Trace metal biogeochemistry
Updated:
Trace metals play crucial roles as micronutrients, influencing biological productivity and ecosystem dynamics, yet their distributions are controlled by a combination of physical, chemical, and biological processes. Biological uptake drives trace metal cycling, as phytoplankton assimilate essential metals like Fe, Zn, Cu, Ni, and Co in the surface ocean, which are subsequently regenerated through microbial decomposition and remineralization processes in the deep ocean. Organic complexation regulates the bioavailability of many trace metals, with strong ligand binding stabilizing dissolved species and extending their residence times. Scavenging onto sinking particles further removes trace metals from seawater, with rates influenced by particle composition and flux. Understanding and quantifying these processes is critical for predicting how marine ecosystems will respond to environmental changes. By integrating numerical modeling with observational datasets, I aim to unravel these interconnected mechanisms and their implications for ocean biogeochemistry, carbon cycling, and climate feedbacks.
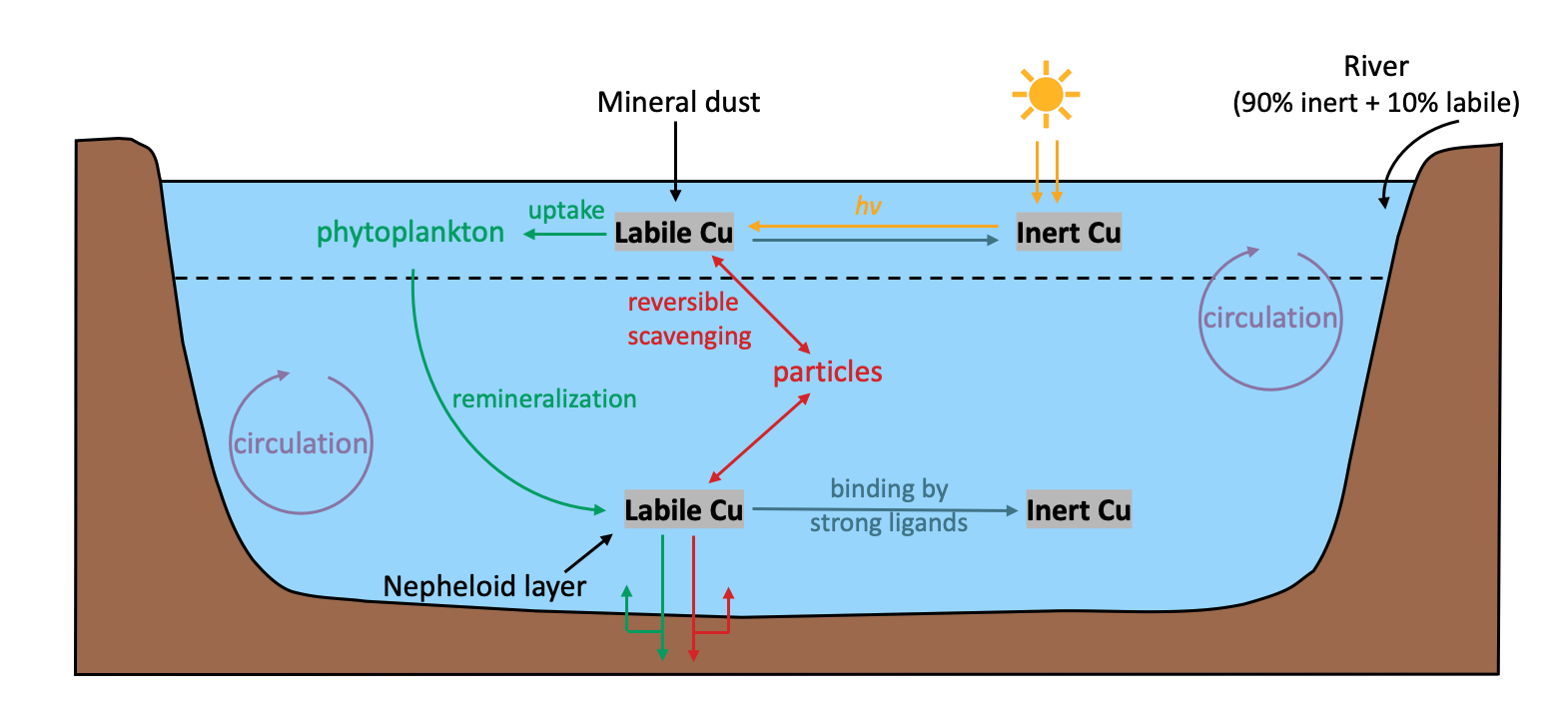 Figure 1 Key processes in the marine biogeochemical cycling of copper (Cu). The dashed black line indicates the base of the euphotic zone. Total dissolved Cu comprises kinetically labile Cu and inert Cu pools. The labile Cu is actively involved in biogeochemical processes, including biological uptake by phytoplankton followed by remineralization (green arrows), and reversible scavenging onto particles (red arrows). Upon reaching the seafloor, a fraction of the biogenic and scavenged labile Cu remineralizes, while the remaining fraction is buried in the sediments. Labile Cu is converted to inert Cu by strong organic ligand binding throughout the water column, while inert Cu is converted back to labile Cu in the euphotic zone through photodecomposition. External sources of Cu to the ocean include rivers, dust, and a dissolved flux coming from the sediment.
Figure 1 Key processes in the marine biogeochemical cycling of copper (Cu). The dashed black line indicates the base of the euphotic zone. Total dissolved Cu comprises kinetically labile Cu and inert Cu pools. The labile Cu is actively involved in biogeochemical processes, including biological uptake by phytoplankton followed by remineralization (green arrows), and reversible scavenging onto particles (red arrows). Upon reaching the seafloor, a fraction of the biogenic and scavenged labile Cu remineralizes, while the remaining fraction is buried in the sediments. Labile Cu is converted to inert Cu by strong organic ligand binding throughout the water column, while inert Cu is converted back to labile Cu in the euphotic zone through photodecomposition. External sources of Cu to the ocean include rivers, dust, and a dissolved flux coming from the sediment.
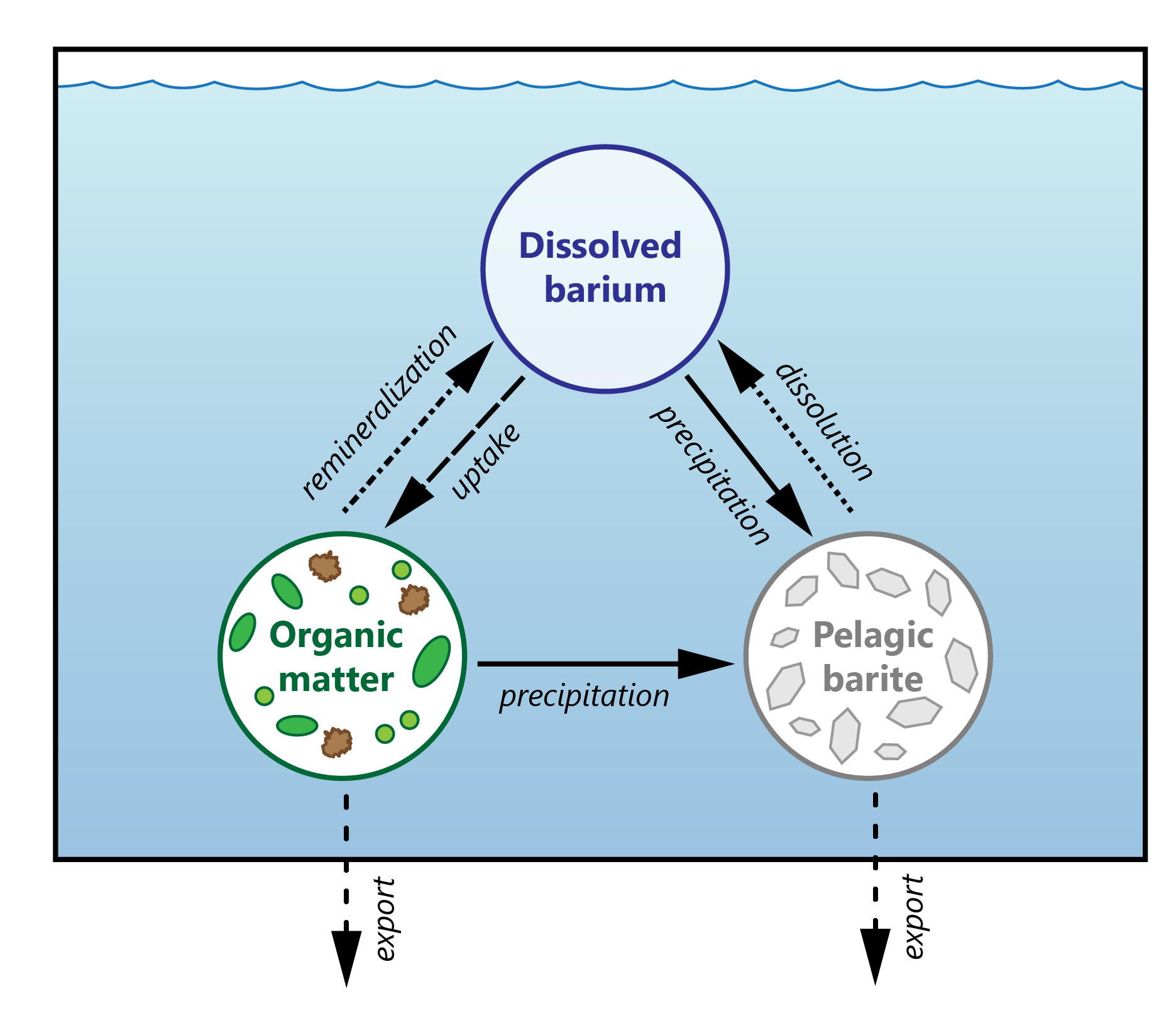 Figure 2 The marine barium(Ba) cycle, illustrating the exchanges between dissolved and particulate phases, with the latter being further separated into pelagic barite and organic matter-associated Ba. Arrows represent key biogeochemical processes regulating these exchanges: solid arrows denote barite precipitation, dotted arrows indicate barite dissolution, the long-dashed arrow represents biological uptake of dissolved Ba from seawater, the dash-dot arrow signifies organic matter remineralization, and the short-dashed arrows indicate particulate Ba export.
Figure 2 The marine barium(Ba) cycle, illustrating the exchanges between dissolved and particulate phases, with the latter being further separated into pelagic barite and organic matter-associated Ba. Arrows represent key biogeochemical processes regulating these exchanges: solid arrows denote barite precipitation, dotted arrows indicate barite dissolution, the long-dashed arrow represents biological uptake of dissolved Ba from seawater, the dash-dot arrow signifies organic matter remineralization, and the short-dashed arrows indicate particulate Ba export.
