Constraining the ocean CaCO3 export and dissolution through a global alkalinity model
Updated:
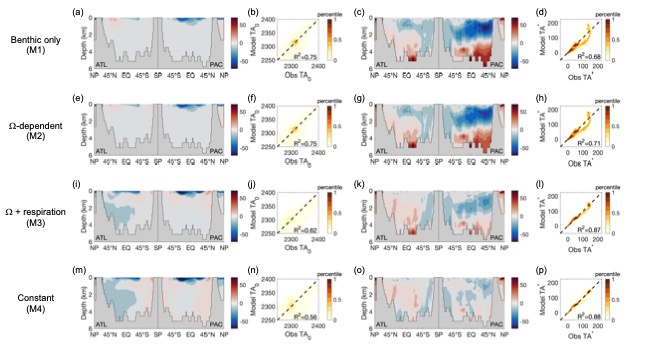
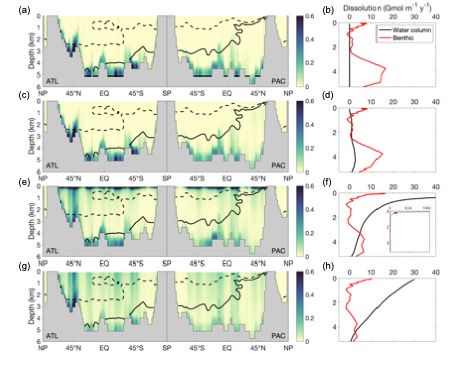
Ocean alkalinity plays a fundamental role in the apportionment of CO2 between the atmosphere and the ocean. The primary driver of the ocean’s vertical alkalinity distribution is the formation of calcium carbonate (CaCO3) by organisms at the ocean’s surface, and its dissolution at depth. This so-called “CaCO3 counterpump” is poorly constrained, however, both in terms of how much CaCO3 is exported from the surface ocean, and at what depth it dissolves. Here, we created a steady-state model of global ocean alkalinity using Ocean Circulation Inverse Model (OCIM) transport, biogeochemical cycling, and field-tested calcite and aragonite dissolution kinetics. We find that limiting CaCO3 dissolution to below the aragonite and calcite saturation horizons cannot explain excess alkalinity in the upper ocean, and that models allowing dissolution above the saturation horizons best match observations. Linking dissolution to organic matter respiration, or imposing a constant dissolution rate both produce good model fits. Our best performing models require export between 1.1 and 1.8 Gt PIC y-1 (from 73 m), but all converge to 1.0 Gt PIC y-1 export at 279 m, indicating that both high- and low-export scenarios can match observations, as long as high export is coupled to high upper ocean dissolution. These results demonstrate that dissolution is not a simple function of seawater CaCO3 saturation (Ω) and calcite or aragonite solubility, and that other mechanisms, likely related to the biology and ecology of calcifiers, must drive significant dissolution throughout the entire water column.